Abstract

Introduction: T-cell prolymphocytic leukemia (T-PLL) is a rare mature T-cell lymphoma with a dismal prognosis. Treatment options for T-PLL are extremely limited. Alemtuzumab is the mainstay of the first-line treatment with a progression-free survival (PFS) of approximately one year. Eligible patients are offered allogeneic hematopoietic cell transplantation (allo-HCT) in consolidation as the only curative approach. Autologous hematopoietic transplantation (auto-HCT) is cited as an option in T-PLL management, e.g., (Staber, Blood 2019; Dearden, Hematology Am Soc Hematol Educ Program 2015), however, extremely scarce data is available on this therapeutic option (Krishnan, Br J Haematol 2010; Dearden, Blood 2001). We hereby present characteristics and outcomes in a cohort of patients with T-PLL undergoing auto-HCT.
Methods: Patients aged at least 18 years old with a T-PLL diagnosis who underwent an auto-HCT between 2000 and 2019 were selected from the EBMT database. Additional data to verify the diagnosis (e.g., immunophenotype, cytogenetics, molecular markers), as well as clarification on treatment prior to and post auto-HCT, HCT-CI score, and cause of death, was requested from participating centres. Overall survival (OS) and PFS were analysed using Kaplan-Meier methods. Cumulative incidence of relapse (RI) and non-relapse mortality (NRM) were analysed together in a competing risks framework. Prognostic factors (sex, interval from diagnosis to auto-HCT, Karnofsky performance score (KPS), number of lines of prior therapy, use of TBI and calendar year, disease status, and age at auto-HCT) were evaluated in univariate analyses.
Results: Forty patients from 31 centers were included out of 42 initially identified. Two patients were excluded based on immunophenotype (positive CD57 or CD34). Additional data was obtained for 19 patients. Median age at auto-HCT was 62 years (IQR 53-67) with males accounting for 58% of the cohort. Median interval between diagnosis and auto-HCT was 8.8 months (IQR 6.4-17.7), and the median calendar year of auto-HCT was 2011 (IQR 2007-2014). A total of 22% of patients had a KPS of ≤80% and 27% an HCT-CI score of 3 or more. Regarding lines of therapy, 80% received only 1 previous therapy and 96% had been exposed to alemtuzumab. At time of auto-HCT, 68% were in complete remission (CR) and 25% in partial remission (PR).
Conditioning was chemotherapy-based in the majority with TBI administered to 10%, at an average dose of 10.4-13 Gy. Engraftment was achieved in all analysed patients. CR rate at 100 days post auto-HCT was 88% (95% CI 73-97%).
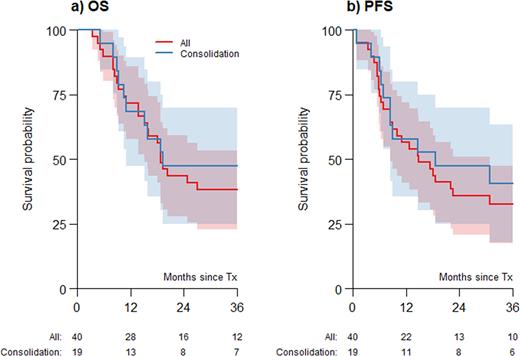
With a median follow-up of 87.7 months (95% CI, 42.3-89.9) the 2-year OS was estimated at 44% (95% CI, 28-59%) (Figure 1a), with a 2-year PFS of 36% (95% CI, 21-51%) (Figure 1b). 2-year RI was 59% (95% CI, 44-75%), while 2-year NRM was 5% (95% CI, 0-12%). None of the prognostic factors evaluated were significantly associated with OS, PFS, RI, or NRM.
For 19 patients (76%), for whom auto-HCT was used as consolidation of response after 1st line alemtuzumab, the CR rate at 100 days after auto-HCT was 85% (95% CI 65-96%). The 2-year estimates for OS and PFS were 47% (95% CI 25-70%) and 47% (95% CI 25-70%), respectively.
For the whole group, progressive disease was the most frequent cause of death (55%). Second malignancies developed in 10% of patients.
Conclusions: Auto-HCT is associated with acceptable efficacy with low NRM and might significantly extend response duration after alemtuzumab. As already suggested in position papers it may represent an important alternative to allo-HCT. Auto-HCT could possibly be used as a tandem option to maintain or increase disease control prior to reduced-intensity allo-HCT.
Figure 1a) Overall survival (OS) and 1b) progression-free survival (PFS) of patients undergoing autologous hematopoietic cell transplantation (auto-HCT). Grey areas represent the 95% confidence intervals. The figures below the graph are the number of patients at risk. The "Consolidation” patients (blue line) are patients with available detailed data receiving auto-HCT as a consolidation of response after first line alemtuzumab. This group is a subset of "All” patients (red line).
Disclosures
Drozd-Sokolowska:Sanofi: Honoraria; AbbVie: Honoraria, Speakers Bureau; Janssen: Honoraria; Servier: Honoraria, Speakers Bureau; Roche: Consultancy. Sierra:Pfizer: Research Funding; Novartis: Honoraria; Jazz: Research Funding. Bermúdez:Bristol Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Speakers Bureau; Neovii: Consultancy, Speakers Bureau; Trecondi: Consultancy, Speakers Bureau; MSD: Consultancy; Pfizer: Consultancy, Honoraria, Speakers Bureau. Sampol:GSK: Consultancy. Wilson:Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants; Celgene: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Tournilhac:Gilead: Honoraria, Other: Travel grant , Research Funding; Securabio: Honoraria, Other: Travel grant , Research Funding; Takeda: Honoraria, Other: Travel grant , Research Funding; Abbvie: Honoraria, Other: Travel grant , Research Funding; Janssen: Honoraria, Other: Travel grant , Research Funding; IdeoGen: Honoraria, Other: Travel grant , Research Funding. McLornan:CELGENE BMS: Research Funding, Speakers Bureau; NOVARTIS: Honoraria, Research Funding, Speakers Bureau; ABBVIE: Speakers Bureau; JAZZ: Honoraria, Speakers Bureau. Yakoub-Agha:Bristol Myers Squibb: Honoraria; Janssen: Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal